Immediate Cord Clamping: the
Primary Injury
Immediate clamping of the umbilical cord before the
child has breathed (ICC) has been condemned in obstetrical literature for over
200 years. [1] [2] In the 1970s, primate research [A][3][4]
using ICC to produce neonatal asphyxia resulted in brain lesions similar to
those of human “neonatal asphyxia.” Two
extensive review papers on placental transfusion and the time of cord clamping
[5,6] both condemned the practice of ICC:
·
Linderkamp ‘82 [5]: “…
immediate cord clamping can cause hypovolemia, hypotension and anemia …”
·
Peltonin ’81 [6]: “Thus
[clamping before the first breath] is unphysiological and should be avoided
under certain unfavourable circumstances the consequences may be FATAL.”
Peltonen described the effect of ICC on cardiac
ventricle filling visualized under fluoroscopy – there was momentary “cardiac
arrest.” This effect is seen in [A] when a normal neonate is subjected to ICC: the
heart rate (AND CARDIAC OUTPUT) fall immediately by about 50%. The umbilical vein is comparable to the vena
cava. Sudden removal of this large
venous return to the heart has major effects on tissue perfusion of the
neonate, as does sudden removal of a very large volume of placental blood from
the general circulation.
However, in the early 1980’s, ICC use increased,
as neonatologists demanded ICC for instant neonatal transport to the
resuscitation table to correct and prevent neonatal asphyxia. Neonatal deaths decreased markedly; the
incidence of cerebral palsy stayed constant.
Lives were saved; brains were not saved.
By the early 1990’s, medico-legal advice encouraged
obstetricians to send an immediately excised portion of cord for blood analysis
[9] to prove that the child was not asphyxiated
at birth. By 2000, ICC was
standard practice. In August
2003, ACOG quietly revoked publication of Practice Bulletin 138;
obstetricians, perinatologists and neonatologists continue to think that ICC is
harmless.
Today, very few neonates are allowed to close the
cord physiologically and to achieve a blood volume that is optimal for
survival; however, the actual amount of individual blood loss from ICC
varies enormously. The child with
intra-partum cord compression (a tight nuchal cord is the most common cause)
combined with ICC [10] is usually critically hypovolemic and prone to HIE,
whereas the child that cries when the head is born, and is delivered with the
next uterine contraction may receive a very adequate (uterine generated) blood
volume before the cord can be clamped.
In general, most vaginally delivered neonates that
breathe before the cord is clamped attain a functional blood volume. Those neonates clamped before the first
breath have less than an optimal blood volume, and preemies, c-section babies,
and depressed babies in this category are prone to severe compromise from
hypovolemia / hypotension.
The pathology generated by ICC also varies widely –
from severe ischemic brain damage and death to none – a normal child. The incidence of cerebral palsy has remained
constant at about 1 per 1000 births over the past 30 years, but an epidemic of
autism, ASD, ADD, ADHD, behavioural and achievement disorders is raging in the
Western World. The specific ischemic
brain lesions related to these “minor” neurological disorders have been
addressed by Eileen Simon [11] and similar disorders have been produced in
primates by birth asphyxia. [3]
Relatively small ischemic lesions at birth in the nuclei of the brain stem that
disrupt function in the auditory / speech circuits may not be apparent until
the child is in school.
In the 1980s, Lozoff published the first correlations
between infant anemia and mental disorders in grade
school children. [7] Multiple publications have confirmed her initial
findings. In 1999, Hurtado showed, in grade
school children, a direct relationship between objective assessments of the
degree of mental deficiency (IQ testing) and the degree of anemia (hemoglobin
levels) in infancy. [8] Non-use of the cord clamp at birth (full placental
transfusion) provides the neonate with enough iron to prevent anemia during the
first year of life. Whether the brain
damage occurs sooner (at birth from hypo-perfusion of the brain) or later (by
affecting brain development), it is preventable by not clamping the cord.
Intra-ventricular hemorrhage (IVH) in preemies is
seen on MRI as a hemorrhagic infarct of the germinal matrix – the most
metabolically active part of the premature brain, manufacturing neurons that
build the cerebral cortex; it is thus the area most susceptible to ischemic
damage. IVH is frequently associated
with IRDS (shock lung) [12] Preemies have routine ICC.
“Sick neonates are one of
the most heavily transfused groups of patients in modern medicine.” [13] “At risk” – sick neonates have routine
ICC. The symptoms and signs of
nearly every child admitted to an NICU – pallor, weakness, inability to suckle,
oliguria / anuria, hypotension, hypothermia, metabolic acidosis, anemia and
hypoglycemia are those of hypovolemia and hypovolemic shock. The retraction respirations (RR) of an ICC
neonate are the same as those of an adult dying in hypovoelmic shock (air
hunger). RR fills the right heart with
blood; it is in response to a very low central venous pressure. Hyaline membranes form in adult shock lung
and neonatal shock lung, IRDS, and in foals, puppies and newborn rabbits that
lose blood volume at birth. [14]
The sequence of intra-partum
Asphyxia-to-HIE-to-Cerebral Palsy has been widely reported, [10] [15] all under
the misconception that hypoxia causes brain damage. The hypoxia resulting from cord compression inevitably entails
fetal hypovolemia – the oxygenated blood engorges the placenta while the fetus
becomes exsanguinated. ICC
finalizes and accentuates the hypovolemic state. Ischemic encephalopathy then begins and
progresses after birth regardless of the oxygenation of the newborn. Macroscopic ischemic neuron necrosis is
readily visualized on MRI. Minor
lesions in small brainstem nuclei are more difficult to define. Physiological neonatal cerebral blood flow
(the MRI norm) following physiological placental transfusion has yet to be
reported.
In summary, ICC may be harmless in the child that
receives a large placental transfusion before the cord is clamped, but ICC
compromises all other neonates to varying degrees by loss of blood volume. Tissue damage results from deficient tissue
perfusion with permanent injury occurring mainly in the brain and lungs,
although multi-organ dysfunction is often seen as well.
The newborn child, premature or otherwise, is very
capable of closing its own cord vessels.
It has had millions of years of natural selection to perfect the
required reflexes that ensure survival.
Very basically, blood volume from the placenta is used to initiate
function of the child’s own life support organs and systems, after which the
placental vessels close reflexively.
The use of a cord clamp during this process disrupts physiology and
causes injury.
Reference:
1.
"A Treatise on the
Management of Pregnant and Lying-In Women" by Charles White, published in
1773.
2.
Erasmus Darwin, Zoonomia, 1801; Vol. III page 321
3.
Windle WF (1969)
Brain damage by asphyxia at birth.
Scientific American 221(#4): 76‑84.
4.
Myers RE (1972) Two
patterns of perinatal brain damage and their conditions of occurrence. American Journal of Obstetrics and
Gynecology 112:246-276.30.
5.
Linderkamp O. Placental
transfusion: determinants and effects. Clinics in Perinatology 1982;9:559-592
6.
Peltonen T. Placental Transfusion, Advantage -
Disadvantage. Eur J Pediatr. 1981;137:141-146
7.
Lozoff B. Jimenez E.
Wolf AW. Long Term Development Outcome
in Infants with Iron Deficiency. N Eng J Med 1991; 325: 687-94.
9.
ACOG Committee Opinion
Number 138 - April 1994, published in the International Journal of Gynaecology
and Obstetrics 45:303-304 [54], reaffirmed 2000, and listed as current in
OBSTETRICS & GYNECOLOGY, February 2002,
10. Frances Cowan et al. Origin and Timing of Brain Lesions in Term Infants with Neonatal Encephalopathy. The Lancet, Vol.361, Issue 9359,1 March, 2003 pages 736-742
11.
Simon E. Brainstem Lesions in
Autism: Birth Asphyxia and Ischemia
as Causative Factors IMFAR presentation October 2002
12. Suarez RD et al. Indomethacin Tocolysis and Intraventricular Hemorrhage. OBSTETRICS & GYNECOLOGY Vol. 97 No. 6 June 2001 921-925.
13.
N A Murray and I A G Roberts. Neonatal transfusion
practice Arch. Dis. Child. Fetal Neonatal Ed., Mar
2004; 89: F101 – 107
15.
Hankins G.D.V. et al.
Neonatal Organ System Injury in Acute Birth Asphyxia Sufficient to
Result in Neonatal Encephalopathy.
OBSTETRICS & GYNECOLOGY, May 2002. Vol. 99, No. 5, part 1. Pages
688-691
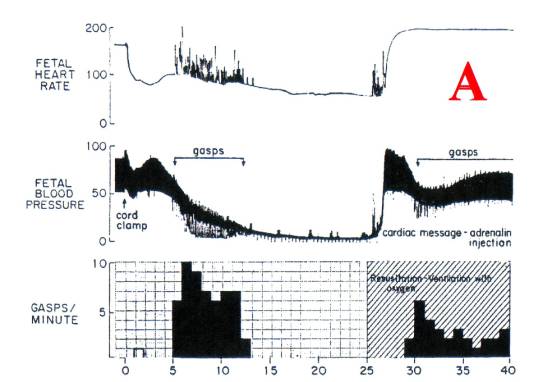
Figure
1. Myers RE (1972) Two patterns of perinatal brain damage and their conditions
of occurrence. American Journal of
Obstetrics and Gynecology 112:246-276.30.
Copyright September 2004 G. M. Morley MB ChB FACOG